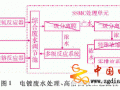
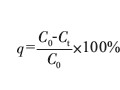摘 要:采用淀粉基黃原酸鹽處理含重金屬的電鍍廢水,對(duì)淀粉基黃原酸鹽的用量,pH值和反應(yīng)時(shí)間等條件進(jìn)行了研究。結(jié)果發(fā)現(xiàn),1L含氰電鍍廢水(含Cr^3+15mg/L,Cu^2+3mg/L,Ni^2+9.2mg/L和Zn^2+6mg/L),加入1g淀粉基黃原酸鹽,調(diào)節(jié)pH為8,攪拌1h,過(guò)濾,處理后的廢水中Cr^3+,Cu^2+,Zn^2+和Ni^2+殘余濃度分別為0.08mg/L,0.01mg/L,0.1mg/L和0.08mg/L。含有重金屬鹽的殘?jiān)捎孟跛崽幚恚曰厥罩亟饘佟?/p>